The Transparency Facade: pharma industry lobbying at the European Commission
The Commission's online transparency of top level meetings only scratches the surface of much more extensive lobbying and industry contacts: Corporate Europe Observatory's exclusive data reveals that the European Commission had up to ten times as many meetings with the pharmaceutical industry at unit level than at top level, in key Commission directorates. These included meetings with industry lobbies that haven't signed up to the Transparency Register. The online disclosure of meetings with commissioners, their cabinets and directors-general shows the public only a slice of the truth, with the bulk of meetings still taking place out of sight and beyond scrutiny.
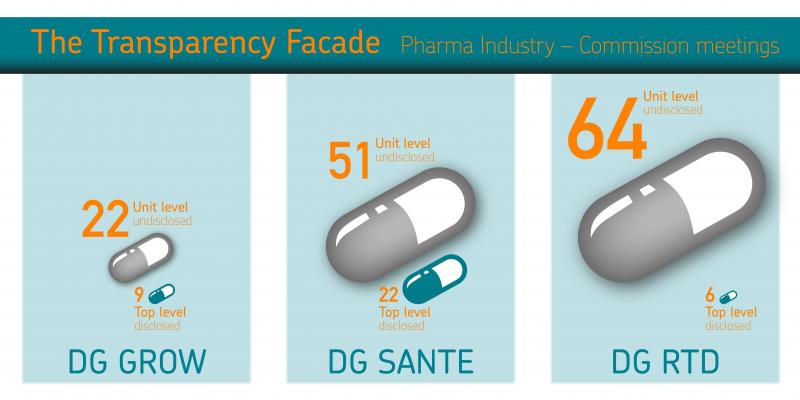
Infographic: Meetings with the pharmaceutical industry in the first four and half months of the Juncker Commission (1 November 2014 to mid-March 2015). Top level refers to meetings at Commissioner, cabinet and Director General level, which are disclosed online. Unit level refers to meetings held with Commission officials within corresponding directorates/units, which are not disclosed to the public - these numbers result from Corporate Europe Observatroy's access to documents requests.
In September this year, CEO published an expose of the big money and close ties behind the pharmaceutical lobby's efforts to influence EU policy, Policy prescriptions: the firepower of the EU pharmaceutical lobby and implications for public health. This was the first of two pieces on Big Pharma's undue influence at EU level. It is extremely important for the public to be able to see the extent to which an industry, especially one whose business has serious implications for the health and well-being of people and planet, is meeting with the policy-makers responsible for regulating it. This is so that citizens can guard against the capture of policy-making by narrow, vested interests ie ensuring that the profit-motivated interests of business and the public-interest motivation of regulators do not become blurred by excessive or imbalanced access to, and input from, a particular industry. Lobbying transparency is a very basic prerequisite for people to be able to see who is seeking to influence the laws that should be made in their interest.
When President Juncker took office, he promised that his Commission would disclose online all meetings with stakeholders held with commissioners, their cabinets, and directors general – in total, less than 300 people. But this leaves the bulk of the Commission (another 30,000+ officials), where most lobbying actually takes place, free to continue to meet and greet as many lobbyists as they wish, with little fear of scrutiny. Subject only to the laws on access to documents, which at best, reveal a limited and piecemeal picture of Commission unit-level lobbying, and consume considerable time and resources of both public interest groups and the Commission itself. The research undertaken for the Policy Prescriptions report is a clear example of this.
As part of the research, access to documents requests were tabled to the Commission's directorates (DGs) most relevant to Big Pharma for lists of meetings held with pharmaceutical industry representatives since the Juncker Commission took office (1 Nov until mid-March 2015, when the requests were tabled). The results of the requests to DG Health and Food Safety (SANTE), DG Research and Innovation (RTD) and DG Internal Market, Industry, Entrepreneurship and SMEs (GROW) showed a remarkable and worrying level of access for the industry. But when compared with the number of meetings with Big Pharma disclosed in the online calendars of the respective commissioners, cabinets and directors-general, the numbers took on another layer of meaning. For the meetings disclosed online with the very top levels of the Commission were vastly outnumbered by those happening at lower levels, which are undisclosed:
DG SANTE had over twice as many meetings with the pharmaceutical industry at unit level (51) than at the top level (22);
DG GROW had over twice as many at unit level (22) than top level (9);
DG RTD had over 10 times more meetings with pharma at unit level (64) than at top level (6)!
These numbers are, moreover, only a minimum and may in fact be higher, as the access to documents requests upon which they are based only concerned some of the directorates and units in each DG (those deemed most relevant to, or most likely targets for, the pharmaceutical industry).1
In the case of DG RTD, Big Pharma enjoys a particularly institutionalised privileged role, through the EU's biggest – and highly problematic - public private partnership IMI (Innovative Medicines Initiative). This multi-billion payout of public money to Big Pharma has had pharma industry lobby group EFPIA (members include pharma giants Novartis, Glaxosmithkline, Pfizer, Bayer etc) in the driving seat since day one. EFPIA has been allowed to set the agenda, goals, grants, and even write the intellectual property regime so they can to capture the profits (see Policy Prescriptions report section 3.3 on IMI for more information).
Furthermore, although the Juncker Commission introduced a de facto ban on high-level meetings with lobbies that haven't signed up to the still-voluntary Transparency Register, it is evident that unregistered industry lobbies are still being met with at unit level. For example, our access to documents revealed that unregistered pharmaceutical companies Almirall, Boehringer Ingelheim and Vifor Pharma all met with DG RTD’s Health directorate,2 whilst unregistered pharmaceutical trade body the Association of Pharmaceutical Research and Development (APRaD) met with DG SANTE.3 CEO repeats ALTER-EU's call upon President Juncker to extend the ban on meeting unregistered lobbyists to all levels of the Commission.
The significantly - and sometimes vastly - larger proportion of meetings taking place with the pharmaceutical industry below the top level of the Commission is a stark illustration of why we need full and proactive online disclosure of all meetings with stakeholders in the Commission, whatever the level. Until then, the progress represented by high-level meeting disclosure, looks rather too much like a token gesture. A gesture which offers the public and press a facade of transparency over who is meeting who and on what topic, but in reality only covers a minority of meetings - as the huge numbers of meetings taking place with Big Pharma at the lower levels of the Commission show.
1The only exception to this pattern was DG Trade, which in response to CEO's access to documents request concerning pharma meetings with DG Trade's Directorates B (Services and Investment, Intellectual Property and Public Procurement), E (Neighbouring countries, USA and Canada) and F (WTO, Legal Affairs and Trade in Goods) only disclosed 3 meetings, whilst around 5 were disclosed online at Trade Commissioner, cabinet and Director General level. This does not however alter the rationale for full disclosure of all meetings.
2DG RTD Directorate E ‘HEALTH’ meeting with EFPIA, GSK, AbbVie, Almirall, AstraZeneca, Bayer, Biogen Idec, Boehringer Ingelheim, GSK, Janssen, MSD, Novartis, Novo Nordisk, Pfizer, Sanofi Pasteur, UCB, Vifor Pharma, 25/02/2015, Brussels, Subject: IMI2 JU.
According to DG RTD response to A2D request GestDem 2015/1628.
3DG SANTE Unit D1 Strategy and inter-national, Unit D5 - Medicinal products, authorisations, European Medicines Agency, Unit D6 - Medicinal products: quality, safety and efficacy, Principal Advisor to the DDG, meeting with EFPIA (European Federation of Pharmaceutical Industries and Associations) and APRaD, Subject: Presentation by EFPIA and APRaD on medicines situation in the Ukraine and their activities there. Short exchange of views of possibilities for DG SANTE to provide help in Ukraine.
According to DG SANTE response to A2D request GestDem 2015/1729.
