
Expanding the corporate capture of research: the new EU Joint Undertakings
The European Commission has proposed to Member States to expand the highly problematic model of EU Joint Undertakings, very large industry-driven public-private research partnerships, in the EU’s new research funding programme Horizon Europe.
Last May 2020, Corporate Europe Observatory exposed how participating corporations had driven two of these partnerships’ research agendas and detailed work programmes in their own interest, rather than the public interest, even not fulfilling their financial commitments in one of the two partnerships.
Now almost €10 billion of EU funding would be up for grabs for nine new partnerships, with no longer any cash contribution foreseen from participating corporations to the projects funded, and a comparable level of influence for them over this money’s uses. Will member states agree to the European Commission’s proposal to keep industry driving the show and the uses of this public research funding, or will they push back?
Almost €10 billion worth of public research funding is proposed by the European Commission for the creation of nine public-private partnerships between the EU and industry. This will fund research projects between 2021 and 2031, and the proposal is currently being examined by the European Council, representing EU member states who will have the last word on the matter.
For the sake of this article we will only examine the issues of agenda-setting (who defines the partnership’s overall research agenda as well as its detailed annual work programmes) and rules around industry’s contributions (who pays what & how much). While an exhaustive analysis of this draft regulation should cover several modifications between the rules defined for the 2014-2020 Joint Undertakings, under Horizon 2020, and those now proposed to EU member states under Horizon Europe for the 2021-2031 period, this however is beyond the current article's remit.
Joint Undertakings in a nutshell
The nine new partnerships created by this regulation would be:
-
the Circular Bio-based Europe Joint Undertaking;
-
the Clean Aviation Joint Undertaking;
-
the Clean Hydrogen Joint Undertaking;
-
the Europe’s Rail Joint Undertaking;
-
the Global Health EDCTP3 Joint Undertaking;
-
the Innovative Health Initiative Joint Undertaking;
-
the Key Digital Technologies Joint Undertaking;
-
the Single European Sky ATM Research 3 Joint Undertaking;
-
the Smart Networks and Services Joint Undertaking.
The new partnerships, all to be headquartered in Brussels, use a type of public-private partnership that has existed since 2014, the 'Joint Undertakings'. Created after intense pressure from corporate lobby groups, these Joint Undertakings are small EU institutions created for ten years by a Council regulation, whose Governing Board is split 50/50 between representatives of the European Commission and industry lobbyists. Between 2014 and 2020, the public financial contribution planned for the seven Joint Undertakings created by the EU was above €7 billion. These structures published several calls for proposals every year, to which applied research consortia including large companies, SMEs and public universities and research centres. The majority of the research consortia selected by Joint Undertakings to obtain the funding were created by companies already participating in the partnerships.
Investing in “key technologies and industries in line with the industrial strategy for Europe”, the purpose of these partnerships as defined by the draft regulation would also be to “develop and accelerate the uptake of innovative solutions throughout the Union” addressing “climate, environmental, health and other global societal challenges contributing to Union strategic priorities, in particular to reach the UNSDGs [United Nations Sustainable Development Goals] and achieve climate neutrality by 2050”. While the aims sound lofty, the devil is in the implementation.
The public research funding at stake for all these new Joint Undertakings is €9.6 billion, to be taken out of the second pillar ('Societal Challenges and Industry Competitiveness' – total budget €53.8 billion) of Horizon Europe (the EU’s Research framework programme 2021-2027). Horizon Europe’s total budget is €95.5 billion, with the first pillar ('Excellent and Open Science' - funding frontier research by universities and public research centres) only totaling €24.9 billion, 26 per cent of the budget, down from 32 per cent in the previous Horizon 2020 EU Research Programme.
Under Horizon 2020, Joint Undertakings were implementing a Strategic Research and Innovation Agenda drafted by industry then adopted by the European Commission. While the EU brought in cash, companies participating in the partnership contributed both financially and in kind, with their own researchers and research facilities. The idea was that Joint Undertakings helped steer industry’s research and development priorities at the European scale, and along societal needs, thanks to public funding. Industry’s in-kind contribution was meant to represent at least the same amount as the EU's funding. The EU funding mostly went to the SMEs and research institutions involved in the projects, while these projects’ outcomes were usually appropriated by the participating companies.
This model for funding R&D is understandably attractive for large companies, as it enables them to get others to perform the research work using public funding, and to reap the rewards without shouldering much of the associated risks. 'De-risking' industry’s research projects is even a stated aim of these partnerships. The burning question is whether this model actually enables additional, riskier research, that these companies would not have performed alone, or whether they simply use this funding opportunity (which they lobbied for) to fund research projects they had planned to invest in anyway. In the latter case, we would be looking at a mere subsidy scheme for large companies, not a policy instrument delivering research results for public needs.
In 2011 the website of the pharmaceutical industry’s lobby group in Brussels, EFPIA (European Federation of Pharmaceutical Industries and Associations), advertised the Innovative Medicines Initiative (IMI) to potentially interested pharma companies by saying that “in some cases, this offers tremendous cost savings, as the IMI projects replicate work that individual companies would have had to do anyway”. This interesting admission was removed from EFPIA’s website, but this situation hardly offers value for EU taxpayers' money! Has the situation improved since this interesting admission? One would need to get a candid response from the companies themselves to be sure, but in our analysis there has been much to be concerned about.

Industry priorities led in IMI and BBI
Our main findings were that the overwhelming majority of the projects we looked at, the very structure and mechanisms of these public-private partnerships, showed that participating companies were controlling the partnerships’ priorities and the use of public EU money for their own direct benefit, de-prioritising the public interest. And this was not only the result of these companies sometimes abusing the partnership, but also a predictable consequence of the way these partnerships were set up and managed. In particular, industry was granted the biggest influence in defining these partnerships’ detailed call for proposals.
Examples of projects funded by these two Joint Undertakings included how a cheaper manufacturing process for a key drug for helping people living with HIV in Africa so far appeared to have only helped Sanofi’s profits, or the chemicals multinational Clariant receiving millions of euros to build a factory to turn enormous amounts of agricultural wheat straw into biofuels (despite the fact that such ‘residues’ have other important uses in farming and are important to nurture soils’ fertility and sink carbon). And this is on top of minutes from an IMI Governing Board meeting which indicated that Big Pharma had opposed a Commission proposal to fund biopreparedness efforts including to speed up vaccine approvals. Projects also included quasi-lobbying initiatives consisting in targeting public regulators with policy proposals, and PR work to win public acceptance for new technologies...
Unease at how these partnerships were being run was also partly reflected in the comments of several member states, who felt they did not have a say in the partnerships’ priorities despite being, through the EU budget, their main funders.
Our investigation recommended against the renewal of these two partnerships, as preliminary elements showed the European Commission was not willing to address their deepest flaws – in particular allowing industry to design these PPPs’ strategic research agendas. Knowing the European Commission wanted to renew them anyway, we also proposed recommendations to remedy some of these PPPs’ flaws in terms of excessive industry influence over annual work programmes, financial abuses by private partners not paying their promised financial contributions, secrecy of key documents, governance shortcomings, and, perhaps most importantly, stopping the corporate capture of public health research resources and limit damages inflicted on climate and biodiversity.
However the European Commission’s DG Research (RTD) and industry lobby groups have been pushing for this policy approach for a long time, and DG RTD doesn’t seem willing (or able) to end the corporate capture of its resources in the name of European industries’ competitiveness and technological innovation. Back in June 2020, a first draft proposal for a new BBI, this time called 'European Partnership for a Circular Bio-based Europe' (CBE), was published, while a draft proposal for a new IMI, this time called 'European Partnership for Innovative Health' (IHI), was published in July 2020.
Déjà vu: industry setting the new research agendas
While in 2014 each Joint Undertaking was created with an individual regulation, this time the European Commission has streamlined a lot of their characteristics and the same regulation is used to create all nine new ones. The regulation has two main parts, the first setting the characteristics common to all Joint Undertakings, while the second contains individual sections detailing each Joint Undertaking’s specific features.
In the section that is common to all Joint Undertakings, the regulation foresees that, as with IMI and BBI, all the Joint Undertakings will implement a Strategic Research and Innovation Agenda (SRIA), ie “the document covering the duration of Horizon Europe that identifies the key priorities and the essential technologies and innovations required to achieve the objectives of a joint undertaking” (Article 2. Definitions, §12), which is drafted by industry. This document, which shall “identify the partnership’s targeted impact, foreseen portfolio of activities, measurable expected outcomes, resources, deliverables, and milestones within a defined timeframe” will be adopted by each Joint Undertaking’s Governing Board, composed 50/50 between the European Commission and industry representatives.
This is a line of thinking in research policy that has been at play at least since the Lisbon Strategy: subsidise industry’s research priorities to help them deliver faster on technologies of societal relevance… except industry itself suggests which specific priorities are of societal relevance. No wonder big business lobby groups like BusinessEurope have been pushing so much for the highest possible amount for Horizon Europe’s budget.
For example, while the decision is still pending, the draft SRIAs for the Circular Bio-based Europe Joint Undertaking (CBE) and the Innovative Health Initiative Joint Undertaking (IHI) have already been sketched out by the relevant lobby groups with a stake. For the CBE, the document was drafted by the Bio-based Industries Consortium (the organisation already representing industry in the BBI), and, for the IHI, the document was drafted by a broader group of corporate lobby groups than for IMI (where only EFPIA was involved): EFPIA (European Federation of Pharmaceutical Industries and Associations), Vaccines Europe (part of EFPIA), Europabio (biotechnology industries), Medtech Europe (medical technology industries), and COCIR (medical imaging, radiotherapy, health ICT and electromedical industries).
Dozens of public health NGOs wrote to the European Commission on this point to warn them that “the private sector is taking the reins on the priority setting of the Public-Private Partnership. Such an approach risks giving priority to commercial strategies, entrenching conflicts of interest and seeing vast amounts of taxpayers’ money diverted to industry’s priorities rather than public health needs, without ensuring sufficient public return on public investment”.
It seems they were ignored, and that moreover letting industry steer the overall priorities for all Joint Undertakings is a policy approach that has now been renewed for all of them.
Who drafts the work programmes?
But beyond the Joint Undertaking’s overall priorities, another key document that industry was able to influence very substantially in the 2014 - 2020 IMI and BBI was the annual work plan (or work programme), which details which specific calls for proposals will be published by the partnership, the financial amounts involved etc. Again, the proverbial devil in the details.
How will this document be drafted and approved in the new partnerships? It is worth pointing that the BBI’s work programme was so influenced by industry – despite formal consultations within the Commission before its adoption – that the BIC (the industry partner in BBI) made it one of the key reasons to join it in this advertisement leaflet (partial screenshot below) to potentially interested companies.
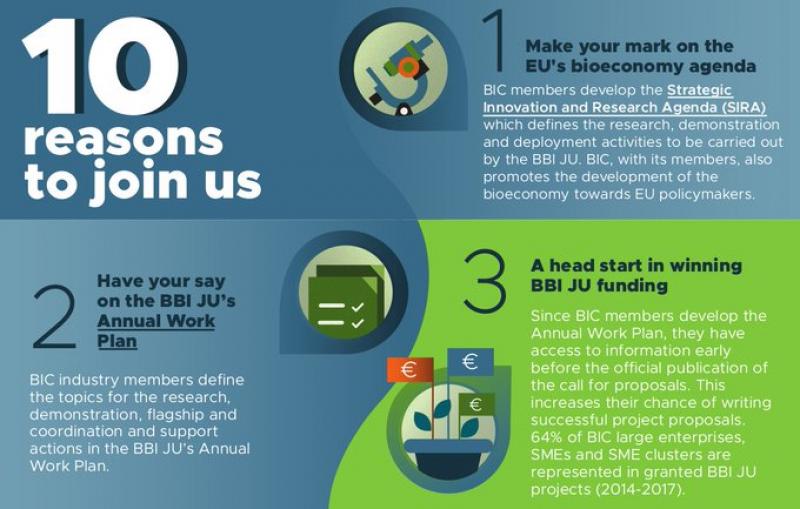
The draft regulation foresees that the new annual work programme will be prepared and submitted by the Executive Director for adoption by the Governing Board. But how will it be drafted, and, crucially, by whom?
The draft text foresees that advisory bodies of the Joint Undertaking, the Scientific advisory body and the States’ representative groups, will provide opinions on it. But the exact procedure for the work programme drafting is not defined in the regulation. The program offices of the Joint Undertakings (the staff running the partnership, who are paid by the EU) have little role in this task, their job being to mainly implement it and check that things are happening according to plans.
A few additional details are provided for some of the Joint Undertakings in the dedicated part of the regulation. For example, the IHI will include an 'Innovation Panel' which will also provide advice on the draft work programme to the Governing Board. Chaired by the Executive Director, its composition will include four Commission representatives, four industry representatives, two states representatives, two “representatives of the scientific community”, and six people appointed later representing an “appropriate representation of stakeholders involved in health care, covering notably the public sector, patients and end-users in general”. Ad hoc panellists (up to six) can also be appointed for each meeting of the panel. But we’re talking about a body which will only meet a couple of times a year.
The general principle is summarised in Recital 21 (emphasis added): “Becoming a founding member or associated member, or one of their constituent or affiliated entities, allows gaining influence, either directly or through the industry representatives, in the governing board of the joint undertaking.… Founding members and associated members and if applicable representing their constituent entities, should therefore be able to contribute to the joint undertaking's agenda and priority setting through the adoption and possible amendment of the Strategic Research and Innovation Agenda, as well as the adoption of the annual work programme, including the content of the calls for proposals, the applicable funding rate per call topic, and the related rules for submission, evaluation, selection, award and review procedures.”
In discussions with European Commission officials, Corporate Europe Observatory has learned that it did “not envisage major changes compared to the well-established process followed under the current generation of Joint Undertakings”, and that “the cooperation between the Joint Undertaking and private partners in drafting the work programmes will remain largely the same”.
In sum, companies involved in the partnership will continue to exert a deep influence on their programming, and the problems found in our investigation in IMI and BBI will be very likely found again in these new partnerships.
Moreover, the annual work programmes for all Joint Undertakings are in fact already being developed, before the regulation’s formal adoption by member states, by industry members of the future Joint Undertakings, industry lobby groups, and the European Commission. The Commission told us: “we are confident that the close interaction between the Commission services, existing JUs and stakeholders during the preparation of the legislative proposal provides a solid basis that will allow for a swift launch of the calls following the adoption of the Council Regulation establishing the JUs under Horizon Europe.”
Our investigations into IMI and BBI revealed that this way of working enabled industry to largely capture the priorities and funding of these two partnerships. Our recommendation was, and remains, that industry partners should of course be consulted during the elaboration of this document, but not given a role in its actual drafting, which should be led by the European Commission, the Scientific Committee, and the States Committee for the public interest to be better defended and reflected in these Joint Undertakings’ priorities.
Finances: will industry manage to get away with not fulfilling its promises again?
One of the most shocking findings of our research into the BBI was that industry was hardly paying any of what it had committed to. Whereas all of the designated projects were supposed to be funded by both public money and industry’s in-kind and financial support, the latest figures available (from the end of 2018 at the time) showed that while the European Commission had already paid 27 per cent (€264.6 million) of their pledged cash contributions, industry partners had so far only paid 3 per cent of theirs (industry was meant to contribute a minimum €182.5 million to the partnership’s operational costs in total) as well as 3.7 per cent per cent of their auditable in-kind contributions. Recently disclosed figures for the BBI’s 2019 budget do not tell a very different story: industry is hardly paying what it had committed to.
Both the BBI office and the European Commission were aware of this, all the more so given that they had pushed the Council to amend the BBI founding regulation that, according to the BIC, made it legally difficult for industry to pay. But the situation did not change once the regulation was changed, and the European Commission did the only thing in its power in such instances: it reduced its funding (by €140 million). The European Court of Auditors said that there was a “high risk that the industry members will not achieve the minimum amount of operational cash contributions by the end of the BBI programme”.
But the situation was uneven across the Joint Undertakings: in IMI, for instance, industry was only meant to contribute in kind to operational costs, and benefited from higher public funding (€1.6 billion). And, as we published, they were lobbying strongly against the very idea of having to contribute financially to the projects.
Not a single Joint Undertaking proposal among the nine provides for a financial contribution by industry to operational costs. The public sector is the only one paying, and moreover sometimes paying twice as with the Key Digital Technologies Joint Undertaking where participating states are to pay a hefty €1.7 billion on top of the €1.8 billion taken from the EU budget. The risk of industry failing to meet its financial commitments seems to have been dealt with – probably helped by industry lobbying – by simple removing industry's obligation to have to contribute financially to projects funded by the Joint Undertakings.
In the draft regulation’s Article 48, the contribution of the CBE private partners is meant to be “at least EUR 1 000 000 000, including up to EUR 23 500 000 for administrative costs”, the share of financial and in-kind contribution not being specified. But the financial details spelled out in the related legislative financial statement tell a different story, with a €23.5 million financial contribution to cover for the partnership’s administrative costs but only €250 million of in kind contributions to operational expenses… while the EU’s financial contribution remains unchanged compared to the BBI, at roughly €1 billion.
But while at first sight this reads as if industry had obtained a spectacular reduction of its commitments, a comment explains that “As BIC did not see the SBA, this cannot be seen as an official and definitive commitment.” In other words, the €250 million figure will change. But the general message is already clear: the European Commission gave up the idea of industry contributing financially to the operational expenses of the CBE Joint Undertaking.
As far as the IHI Joint Undertaking is concerned, the contribution of the private partners is defined as “at least EUR 1 000 000 000, including up to EUR 30 212 000 for administrative costs”. The public sector will contribute €1 billion, a reduced amount compared to IMI (€1.6 billion between 2014 & 2020). Here, the related legislative financial statement plans that industry will indeed bring a financial contribution of €30.2 million for administrative costs, and €1.17 billion of in kind contribution to operational expenses. Here again, industry is not asked to bring any financial contribution to operational expenses.
The draft regulation tries at least to automate and simplify the calculation of industry’s in kind contribution – ie the staff time, facilities etc. This is very important, as our research into IMI in particular (and repeated critical comments by MEPs involved in the budget discharge procedure, the only way the European Parliament can control what Joint Undertakings do) found that, since there was no standard method for reporting or calculating ‘in-kind’ funding from industry, companies had a lot of margin of manoeuvre to claim whatever they wanted as counting towards a contribution to the projects (including even their work as part of the IMI Governing Board!), while opposing disclosure of essential documents such as staff time-sheets.
Conclusion
There would be many more points to discuss to propose a comprehensive assessment of this regulation proposal by the European Commission (our partners for the IMI research, Global Health Advocates, have just published a more detailed analysis of the IHI proposal). But on the two points analysed here, agenda-setting at general and detailed level, and financial contributions, the conclusion is clear: in the Joint Undertakings created by this draft regulation, participating companies would still largely control how the general strategic research agenda and specific annual work programmes are defined, without having to risk any money in the projects’ operational costs. These would be covered by the EU budget entirely.
Subsidising industries along their wishes can sometimes be a legitimate policy choice when these wishes serve the public interest. But these proposed Joint Undertakings leave the definition of the public interest to the private sector, diverting €10 billion and considerable resources from Europe’s public research capacity at a time when, in many EU countries, the working conditions of most young researchers are a scandal and we’ve never needed more to invest into the production of socially relevant knowledge and technologies in the years to come. It is high time the public sector reclaims the control of its resources for the public interest.
